The Merck Index
Thirteenth Edition
Oil of Cumin
Literature References: Volatile oil from fruit of Cuminum cyminum L., Umbelliferae. Constit. 30-40% cuminaldehyde; p-cymene, b-pinene, dipentene.
Properties: Colorless to yellow liquid. d2525 0.900-0.935. aD20 +4 to +8°. nD20 1.4950-1.5090. Almost insol in water; sol in 10 vols 80% alcohol; more sol in stronger alcohol; very sol in chloroform, ether. Keep well closed, cool and protected from light.
Optical Rotation: aD20 +4 to +8°
Index of refraction: nD20 1.4950-1.5090
Density: d(25°C/25°C) 0.900-0.935
Use: Flavoring in Indian curry powder.
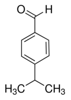
Cuminaldehyde
CAS Registry Number: 122-03-2
CAS Name: 4-(1-Methylethyl)benzaldehyde)
Additional Names: p-isopropylbenzaldehyde; cuminal; cumaldehyde
Molecular Formula: C10H12O
Molecular Weight: 148.20.
Percent Composition: C 81.04%, H 8.16%, O 10.80%
Line Formula: (CH3)2CHC6H4CHO
Literature References: Constituent of eucalyptus, myrrh, cassia, cumin, and other essential oils; prepared synthetically from p-isopropylbenzoyl chloride. Alternate synthesis by formylation of isopropylbenzene under pressure: Crounse, J. Am. Chem. Soc. 71, 1263 (1949). Toxicity study: P. M. Jenner et al., Food. Cosmet. Toxicol. 2, 327 (1964).
Properties: Colorless to yellowish, oily liq; strong persistent odor; acrid, burning taste. d20 0.978. bp760 235-236°; bp35 131-135°. nD20 1.5301. Practically insol in water. Sol in alc, ether. LD50 orally in rats: 1390 mg/kg (Jenner).
Boiling point: bp760 235-236°; bp35 131-135°
Index of refraction: nD20 1.5301
Density: d20 0.978
Toxicity data: LD50 orally in rats: 1390 mg/kg (Jenner)
Derivative Type: Thiosemicarbazone
CAS Registry Number: 3811-20-9
Manufacturers' Codes: SHCH-58
Molecular Formula: C11H15N3S
Molecular Weight: 221.33.
Percent Composition: C 59.69%, H 6.83%, N 18.99%, S 14.49%
Literature References: Prepn: P. P. T. Sah, T. C. Daniels, Rec. Trav. Chim. 69, 1545 (1950); J. Bernstein et al., J. Am. Chem. Soc. 73, 906 (1951).
Properties: Stout platelets from methanol, mp 147°. Practically insol in water. Moderately sol in alc.
Melting point: mp 147°
Cymene
CAS Registry Number: 25155-15-1
CAS Name: Methyl(1-methylethyl)benzene
Additional Names: isopropyltoluene; methylisopropylbenzene
Molecular Formula: C10H14
Molecular Weight: 134.22.
Percent Composition: C 89.49%, H 10.51%
Literature References: Usually prepd by alkylation of toluene; m-, o- and p-isomers obtained: Allen, Yats, J. Am. Chem. Soc. 83, 2799 (1961). Purification and properties of the three isomers: Streiff et al., Anal. Chem. 27, 411 (1955). Separation of the three isomers by gas chromatography: Rihani, Froment, J. Chromatog. 18, 150 (1965). Toxicity study: P. M. Jenner et al., Food Cosmet. Toxicol. 2, 327 (1964).
Derivative Type: m-Cymene
CAS Registry Number: 535-77-3
Additional Names: 1-Methyl-3-(1-methylethyl)benzene
Properties: Liquid, bp 175.14°. mp -63.75°. d420 0.8610, d425 0.8570. nD20 1.4930, nD25 1.4906. Practically insol in water. Miscible with alcohol, ether.
Melting point: mp -63.75°
Boiling point: bp 175.14°
Index of refraction: nD20 1.4930; nD25 1.4906
Density: d420 0.8610; d425 0.8570
Derivative Type: o-Cymene
CAS Registry Number: 527-84-4
Additional Names: 1-Methyl-2-(1-methylethyl)benzene
Properties: Liquid, bp 178.15°. mp -71.54°; also reported as -75.24° and -81.53° for two unstable solid forms (Streiff). d420 0.8766, d425 0.8726. nD20 1.5006, nD25 1.4982. Practically insol in water. Miscible with organic solvents.
Melting point: mp -71.54°; also reported as -75.24° and -81.53° for two unstable solid forms (Streiff)
Boiling point: bp 178.15°
Index of refraction: nD20 1.5006; nD25 1.4982
Density: d420 0.8766; d425 0.8726
Derivative Type: p-Cymene
CAS Registry Number: 99-87-6
Additional Names: 1-Methyl-4-(1-methylethyl)benzene; Dolcymene
Properties: Occurs in a number of essential oils. Liquid, bp 177.10°. mp -67.94°. d420 0.8573, d425 0.8533. nD20 1.4909, nD25 1.4885. Flash pt, closed cup: 117°F (47°C). Practically insol in water. Misc with alcohol, ether. LD50 orally in rats: 4750 mg/kg (Jenner).
Melting point: mp -67.94°
Boiling point: bp 177.10°
Flash point: Flash pt, closed cup: 117°F (47°C)
Index of refraction: nD20 1.4909; nD25 1.4885
Density: d420 0.8573; d425 0.8533
Toxicity data: LD50 orally in rats: 4750 mg/kg (Jenner)
beta-Pinene
CAS Registry Number: 127-91-3
CAS Name: 6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptane
Additional Names: nopinene
Molecular Formula: C10H16
Molecular Weight: 136.23.
Percent Composition: C 88.16%, H 11.84%
Literature References: Found in most essential oils which contain a-pinene, but in far smaller proportions; the l-form occurs most commonly. Initial identification: A. von Baeyer, Ber. 29, 25 (1896). Isoln of the d-form from Ferula galbaniflua Boiss. et Buhse, Umbelliferae: B. N. Rutovski, I. V. Vinogradova, J. Prakt. Chem. 120, 41 (1928); from Cynomarathrum nuttallii A. Gray, Umbelliferae: E. K. Nelson, J. Am. Chem. Soc. 55, 3400 (1933). Irreversible isomerization of b-pinene to a-pinene occurs on shaking with platinum black satd with hydrogen: F. Richter, W. Wolff, Ber. 59, 1733 (1926). Synthesis: G. Bonnet, Bull. Inst. Pin 1938, 217; 1939, 1, C.A. 33, 42234 (1939); K. J. Crowley, Proc. Chem. Soc. (London) 1962, 245; Tetrahedron 21, 1001 (1965); L. M. Harwood, M. Julia, Synthesis 1980, 456. For general refs see a-pinene.
Derivative Type: dl-Form
Properties: bp760 165-166°.
Boiling point: bp760 165-166°
Derivative Type: d-Form
Properties: bp760 164-166°. d2020 0.8654. nD20 1.4739. [a]D +28.59° (Nelson). Also reported as bp760 162-163°. d2020 0.8662. nD20 1.4745. [a]D +20.75° (Rutovski, Vinogradova).
Boiling point: bp760 164-166°; bp760 162-163°
Optical Rotation: [a]D +28.59° (Nelson); [a]D +20.75° (Rutovski, Vinogradova)
Index of refraction: nD20 1.4739; nD20 1.4745
Density: d2020 0.8654; d2020 0.8662
Derivative Type: l-Form
Properties: bp760 162-163°. d15 0.874. nD15 1.4872, [a]D -22.4°.
Boiling point: bp760 162-163°
Optical Rotation: [a]D -22.4°
Index of refraction: nD15 1.4872
Density: d15 0.874
 alimentata da acqua corrente. Io ho utilizzato una pompa di riciclo.
Per distillare piante e oli particolarmente sensibili e fare una vera e propria distillazione in corrente di vapore, si interpone una colonna tra la caldaia e la testa, da caricare con il materiale vegetale, tenuto lontano dall'acqua in ebollizione da un'apposita griglia. Io non l'ho utilizzata.
Inoltre, alla base della caldaia, è buona pratica porre un filtro a purea in modo da evitare che i residui vegetali si brucino e restino attaccati al fondo, inconveniente comunissimo quando si lavora con vetreria classica da laboratorio.
Una volta ottenuto l'olio essenziale di semi di cumino ho estratto e purificato la cuminaldeide in esso contenuta, usando la patriottica Reazione di Bertagnini.
MATERIALE:
- Distillatore in rame
- Semi di cumino
- Etere di petrolio 40-60
- Solfato di magnesio anidro
- Metabisolfito di sodio
- Etanolo 98%
- Acido cloridrico 10%
- Carbonato di sodio
alimentata da acqua corrente. Io ho utilizzato una pompa di riciclo.
Per distillare piante e oli particolarmente sensibili e fare una vera e propria distillazione in corrente di vapore, si interpone una colonna tra la caldaia e la testa, da caricare con il materiale vegetale, tenuto lontano dall'acqua in ebollizione da un'apposita griglia. Io non l'ho utilizzata.
Inoltre, alla base della caldaia, è buona pratica porre un filtro a purea in modo da evitare che i residui vegetali si brucino e restino attaccati al fondo, inconveniente comunissimo quando si lavora con vetreria classica da laboratorio.
Una volta ottenuto l'olio essenziale di semi di cumino ho estratto e purificato la cuminaldeide in esso contenuta, usando la patriottica Reazione di Bertagnini.
MATERIALE:
- Distillatore in rame
- Semi di cumino
- Etere di petrolio 40-60
- Solfato di magnesio anidro
- Metabisolfito di sodio
- Etanolo 98%
- Acido cloridrico 10%
- Carbonato di sodio









 I 5 ml che ho scritto dopo erano semplicemente la resa di aldeide impura.
Dopo la seconda purificazione sono diventati 1,1 grammi.
La resa di aldeide in effetti è bassina, ma, considerando i volumi in gioco e la procedura relativamente improvvisata, è accettabile.
Spero di aver capito bene cosa intendesse...
I 5 ml che ho scritto dopo erano semplicemente la resa di aldeide impura.
Dopo la seconda purificazione sono diventati 1,1 grammi.
La resa di aldeide in effetti è bassina, ma, considerando i volumi in gioco e la procedura relativamente improvvisata, è accettabile.
Spero di aver capito bene cosa intendesse...
